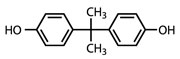
The US Food and Drug Administration (FDA) says there is insufficient scientific evidence to ban bisphenol A (BPA) in food packaging. The agency's announcement came in response to a petition from the Natural Resources Defense Council (NRDC) seeking to prohibit the use of the controversial endocrine disruptor in food contact materials.
The FDA has decided that the best course of action is to continue its review of emerging data on BPA. Agency spokesperson Doug Karas, emphasised that the agency has made no final safety determination on BPA.
 |
BPA is used as a lining in tin cans to prevent food reacting with the metal
|
Although the FDA acknowledges that studies have linked BPA with a variety of health problems, the agency says serious questions remain about the research. According to the FDA, the overwhelming majority of these studies cannot be used to assess the safety of BPA. These limitations include non-oral administration of BPA to animals, which means findings cannot be directly equated to dietary consumption.
Furthermore, the FDA says its research shows that exposure to dietary BPA for infants is much lower than previously estimated. The agency's initial exposure estimates were 2.42 µg/kg of body weight per day (µg/kg-bw/day) for infants and 0.185 µg/kg-bw/day for adults. But its revised estimate is 0.2-0.4 µg/kw-bw/day for infants and 0.1-0.2µg/kg-bw/day for children and adults.
Unsurprisingly, the American Chemistry Council (ACC) celebrated the FDA's announcement, while the NRDC and other environmental groups were disappointed.
'We are encouraged by the FDA's decision,' says ACC spokesperson Scott Jensen. 'It does show the research and studies to date support the continued use of BPA in food contact uses.' Jensen applauds the FDA for an 'objective review' of the existing studies on BPA, which revealed their shortcomings.
NRDC's senior scientist Jennifer Sass emphasises that dozens of animal studies have shown BPA's harmful health effects at low doses and epidemiology studies suggest that human exposure to the compound can cause cardiovascular problems, as well as obesity and metabolic changes that could lead to diabetes. The chemical has also been linked to breast cancer, prostate cancer and reproductive problems. 'The FDA is out of step with the level of safety that consumers want and they are out of step with what the majority of scientists are saying,' Sass tells Chemistry World.
 |
BPA has been linked to cardiovascular problems, diabetes, cancer and other illnesses
|
Nevertheless, Scott Belcher - a pharmacologist at the University of Cincinnati, US, who has studied BPA's health effects - calls the FDA's determination 'reasonable', given that the agency is awaiting results from relevant government-funded studies. He adds that it doesn't make sense for FDA to rule on BPA safety when its own studies are ongoing.
The FDA is slated to complete another updated safety review of BPA this year. The agency is also working with the National Institute of Environmental Health Sciences on BPA, which has invested $30 million (£19 million).
Other jurisdictions have already taken action against BPA. Baby bottles containing BPA have been banned in the EU for more than a year, and Canada and China have similar bans. In the absence of FDA action, 11 US states have also banned the sale of baby bottles with BPA, and some also prohibit children's food or toy packaging with the compound.
Source: Royal Society of Chemistry
No comments:
Post a Comment