Electrolysis is a chemical process that uses electricity to cause a non-spontaneous redox reaction to occur.
2. Electrolytic cell
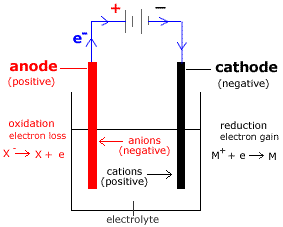
(a) Made up of two electrodes connected to a battery immersed in an electrolyte.
(b) Battery functions as a source of direct current that forces electrons out of anode and pushes them towards the cathode.
(c) Electrolyte contains positively charged ions (cations) and negatively charged ions (anions). It is a compound that conducts electricity. Thus, it must be in either molten salts or aqueous solution of salts, due to presence of freely move anions and cations.
3. Operation of an electrolytic cell
(a) At anode- Anions move to the anode, lose their electrons and oxidise to atoms.
- Oxidation reactions take place at anode.
- Positive electrode in electrolytic cell.
- Cations move to the cathode, gain the electrons and reduce to atoms.
- Reduction reactions take place at cathode.
- Negative electrode in electrolytic cell.
4. Factors influencing the selective discharge of a species at the electrode
(a) Standard reduction/electrode potential of the species
- At cathode, if there is more than one reducible species in the solution, the species with the more positive Eo is preferentially reduced.
2 H2O (l) + 2e- → H2 (g) + 2 OH- (aq) Eo = -0.83 V
Since the Eo value is more positive for H2O water, H2O water is preferentially reduced than Na+.
- At anode, if there is more than one oxidisable species in the solution, the species with the more negative Eo is preferentially oxidised.
Br2 (l) + 2e- → 2 Br- (aq) Eo = +1.07 V
Since the Eo value is more negative for I- ion, I- ion is preferentially oxidised than Br- ion.
(b) Concentration of the species
- The increase of concentration of an ion in a solution tends to promote its discharge.
- For example, the standard reduction potential for calcium ions (Ca2+) and magnesium ions (Mg2+) are quite close.
Ca2+ (aq) + e- → Ca (s) Eo = -2.87 V
The higher concentration of Ca2+ ions in the solution will promote its discharge.
(c) Nature of electrodes
- Inert electrodes do not participate in the redox reactions of the cell. For example, graphite and platinum.
- Active electrodes participate in the redox reactions of the cell. For example, the use of copper electrodes in the electrolysis of aqueous solution of copper (II) sulphate (CuSO4).
5. Electrolysis of molten salts
- Salts contain cations and anions attracted to each other by strong ionic bonds. In the solid state, these ions are unable to move. Solid salts are thus non-electrolytes.
- Salts become electrolytes above their melting points because in the molten state, the ions are able to move (or freely move).

- Molten NaCl contains Na+ and Cl- ions.
- At anode, Cl- ions are oxidised to chlorine atoms. Two chlorine atoms are formed and combine to give Cl2 gas.
- At cathode, Na+ ions are reduced to sodium atoms.
- The half-cell equations:
Cathode: 2 Na+ (l) + 2e- → 2 Na (s)
- The electrolysis of molten NaCl gives sodium metal at the cathode and chlorine gas at the anode.
6. Electrolysis of water
- At anode, water is oxidised to oxygen gas.
- At cathode, water is reduced to hydrogen gas
- The half-cell equations and overall cell reaction equation:
Cathode: 4 H2O + 4e- → 2 H2 (g) + 4 OH- (aq)
Overall reaction: 6 H2O → 2 H2 (g) + O2 (g) + 4 H2O
2 H2O → 2 H2 (g) + O2 (g)
- For 2 water molecules, the volume of hydrogen and oxygen gas formed are in the ratio of 2:1.
7. Electrolysis of aqueous solution
- Water-soluble salts form aqueous solution containing cations and anions from the salt and water molecules.
- Water is an electro-active substance. It can be oxidised or reduced in an electrochemical process, depending on the conditions of electrolysis.
Oxidation: 2 H2O → O2 (g) + 4 H+ (aq) + 4e- Eo = -1.23 V
(a) Electrolysis of dilute aqueous solution of sodium chloride (NaCl)
- Aqueous NaCl solution contains Na+ ions, Cl- ions and water molecules.
2 H2O + 2e- → H2 (g) + 2 OH- (aq) Eo = -0.83 V
- At cathode, water molecules are preferentially reduced than Na+ ions due to its more positive Eo value. Thus, hydrogen gas is formed at the cathode.
O2 (g) + 4 H+ (aq) + 4e- → 2 H2O (g) Eo = +1.23 V
- At anode, water molecules are preferentially oxidised than Cl- ions due to its more negative Eo value. Thus, oxygen gas is formed at the anode.
- The half-cell equations and overall cell reaction equation:
Cathode: 4 H2O + 4e- → 2 H2 (g) + 4 OH- (aq)
Overall reaction: 6 H2O → 2 H2 (g) + O2 (g) + 4 H2O
2 H2O → 2 H2 (g) + O2 (g)
(b) Electrolysis of concentrated aqueous solution of sodium chloride (NaCl)

- Aqueous NaCl solution contains Na+ ions, Cl- ions and water molecules.
2 H2O + 2e- → H2 (g) + 2 OH- (aq) Eo = -0.83 V
- At cathode, water molecules are preferentially reduced than Na+ ions due to its more positive Eo value. Thus, hydrogen gas is formed at the cathode.
- At anode, Cl- ions are preferentially oxidised than water molecules due to its higher concentration. The higher concentration of Cl- ions in the solution will promote its discharge. Moreover, the rate of oxidation of Cl- ions is faster than rate of oxidation of water molecules. Thus, chlorine gas is formed at the anode.
- The half-cell equations and overall cell reaction equation:
Cathode: 2 H2O + 2e- → H2 (g) + 2 OH- (aq)
Overall reaction: 2 Cl- (aq) + 2 H2O → H2 (g) + Cl2 (g) + 2 OH- (aq)
- The Na+ ions and OH- ions left in the electrolytic bath form NaOH, which is actually the intended chemical in the electrolysis of aqueous solution of NaCl.
(c) Electrolysis of aqueous solution of sodium sulphate (Na2SO4)
- Aqueous Na2SO4 solution contains Na+ ions, SO42- ions and water molecules.
2 H2O + 2e- → H2 (g) + 2 OH- (aq) Eo = -0.83 V
- At cathode, water molecules are preferentially reduced than Na+ ions due to its more positive Eo value. Thus, hydrogen gas is formed at the cathode.
- At anode, water molecules are preferentially oxidised than SO42- ions because SO42- ions cannot be oxidised. The sulphur atom in the anion is already at the maximum oxidation state of +6. Thus, oxygen gas is formed at the anode.
- The half-cell equations and overall cell reaction equation:
Cathode: 4 H2O + 4e- → 2 H2 (g) + 4 OH- (aq)
Overall reaction: 6 H2O → 2 H2 (g) + O2 (g) + 4 H2O
2 H2O → 2 H2 (g) + O2 (g)
- The overall reaction does not actually involve Na+ ions and SO42- ions. These ions are used to increases the rate of electrolysis, in increasing the mobility of the ions present.
8. Faraday's first law of electrolysis
Faraday's first law of electrolysis states that the quantity (or mass) of a substance formed at an electrode is directly proportional to the quantity of electric charge that has flowed through the circuit.
Quantity of electric charge, Q = I t
where I = current (A or Cs-1)
t = time (s)
The charge on one mole of electron is 1 faraday (F) where 1 mol electron = 1 F = 96500 C
2 comments:
This was a fantastic blog. A lot of very good information given,
Sell Ku RC Crystal on Line,
Purchase Yellow Research Chemical Powder in Florida
This is really interesting. Very useful information has been conveyed in this article! Thanks for sharing.
Post a Comment