Chemical reaction can proceed in either non-reversible or reversible reaction.
(a) Non-reversible reaction
Reaction which proceed in only one direction.
Example: CaCO3 (s) → CaO (s) + CO2 (g)
(b) Reversible reaction
Reaction which can go in both forward and reverse direction.
Example: N2O4 (g) ↔ 2 NO2 (g)
Forward reaction: Proceeds from left to right.
Reverse reaction: Proceeds from right to left.
The sign (↔) is used to indicate a reversible reaction.
2. Dynamic equilibrium
Consider a reversible reaction: A + B ↔ C + D
(a) As the reaction progresses,
- Concentration of reactants, [A] & [B] decreases with time.
- Concentration of products, [C] & [D] increases with time.
- The concentration of reactants and products remains constant (or unchanged).
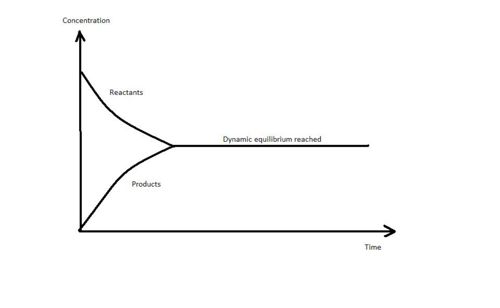
- The rate of forward reaction is equal to the rate of reverse reaction, but the reaction is not stop.
- At this moment, a system is said to be in dynamic equilibrium.

3. Characteristics of a system in equilibrium
(a) The concentration (or pressure) of reactants and products remains constant (or unchanged) over time.
(b) The rate of forward reaction is equal to the rate of reverse reaction (both forward and reverse reaction proceed at an equal rate).
(c) Reaction quotient (Q) is equal to the equilibrium constant (K).
 |
| Rate of water flow in = Rate of water flow out (Dynamic equilibrium) |
4. Law of mass action (Equilibrium law)
For a reversible reaction which is at equilibrium, the ratio of the concentration of reactants and products (both raised to the power of their stoichiometric coefficient) is a constant at a given temperature.
Consider a general reaction: aA + bB ↔ cC + dD
At equilbrium,
 where K = equilibrium constant
where K = equilibrium constant
No comments:
Post a Comment