Researchers in the UK and the US claim to have discovered a new phase of hydrogen in which the diatomic molecules break apart to form six-atom rings, similar to graphene. The new phase, which occurs at very high pressures, could be a stepping stone towards a long-sought after phase: metallic hydrogen.
The quest for metallic hydrogen has been on since the late 19th century, when chemists pointed out that the element, which tops the periodic table's column of alkali metals, ought to form a metal. In 1935, physicists Eugene Wigner and Hillard Bell Huntington predicted that hydrogen should become a metallic solid at high pressures - roughly 25 GPa - but experiments later performed at these pressures showed no trace of a metal transition. More recent experiments have employed higher pressures still. Indeed, last year Mikhail Eremets and Ivan Troyan of the Max-Planck Institute for Chemistry in Mainz, Germany, claimed evidence for metallic hydrogen at pressures up to 260 GPa. But other scientists believed that evidence was unreliable.
The challenge of metallic hydrogen is alluring because it has the potential for significant applications. For example, some believe studies of the material could lead to a room temperature superconductor, which would enable lossless power transmission.
 |
| Under intense pressures hydrogen can form a structure similar to graphene |
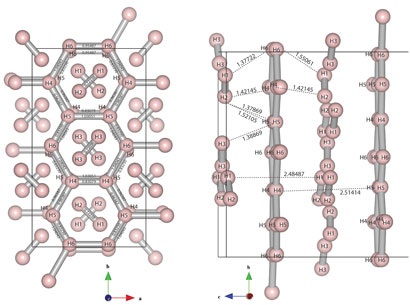
Now, Eugene Gregoryanz of the University of Edinburgh and colleagues have uncovered a new phase of solid hydrogen, which they say might be a stepping stone to the metallic phase. There are three known solid phases of hydrogen which can be created by supercooling the gas: phase I, a close-packed structure of freely rotating molecules; phase II, a similar structure but with some degree of orientational order; and phase III, a structure in which the strength of the H-H bonds turns so weak that the hydrogen could be considered partly atomic, not molecular. The area around the critical point on the phase diagram where these three phases intersect is well mapped out, but no-one has been sure what happens beyond phase III, at higher pressures. So this is where Gregoryanz's group has turned its attention.
The researchers subjected samples of hydrogen and deuterium to pressures up to 315 GPa in a diamond anvil cell at temperatures of 300 K. Using Raman spectroscopy, they measured the vibron frequency, which determines the strength of the H-H bonds and which therefore describes how 'molecular' the hydrogen is. At 220 GPa, the researchers found that the main vibron frequency rapidly decreased, while a second vibron appeared, maintaining the original frequency.
To understand these results, Gregoryanz's group turned to a theory of solid-hydrogen phases published in 2007 by physicists Chris Pickard and Richard Needs in the UK. One of the physicists' predictions for a new phase closely matched the latest results: graphene-like layers of hydrogen in irregular six-atom rings, which would explain the low vibron frequency, interspersed with unbound hydrogen molecules, which would explain the second, higher vibron frequency. At higher pressures, Gregoryanz says, these graphene-like layers may become symmetrical, and exhibit semi-metallic behaviour.
William Nellis, a leader in the search for metallic hydrogen at Harvard University, US, calls the work a 'major revitalisation' of the study of dense hydrogen. He says the electronic band-gap of 1.8 eV measured by the researchers is still very large compared with that required for a metal, but thinks the experimental techniques will pave the way for further study. 'They reported details of how they made their gaskets to function at such high pressures without hydrogen diffusing out of their diamond anvil cells,' he says. 'This knowledge will be useful to other experimentalists in this field.'
Source: Royal Society of Chemistry
No comments:
Post a Comment