(a) Arrangement of particles
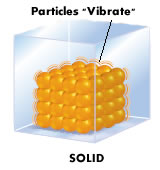
- Solid particles are closely packed together and in a rigid arrangement.
- They are not able to move freely but can only vibrate and rotate about fixed position.
(b) Interparticle bonding or forces
Solid particles are held by strong interparticle forces.
(c) Shape and volume

- Has definite shape and volume.
- It is due to the strong interparticle attractive forces that hold the solid particles firmly in place. Thus, the movement of the particles is restricted to vibration and rotation at its own axis.
(d) Compressibility
Incompressible because there is no empty space between the solid particles.
2. Phase change of matter

3. Melting (Fussion)
(a) Melting is a process in which a substance changes from solid to liquid phase.

(b) When a solid substance is heated:
- Its particles gain energy. Able to vibrate more energetically.
- When it reaches a temperature at which the particles have enough energy to overcome the interparticle attractive forces between themselves, particles are free to move.
- Solid is changing into liquid.
4. Freezing (Solidification)
(a) Freezing is a process in which a substance changes from liquid to solid phase.
(b) When a liquid is cooled:
- The kinetic energy of the liquid molecules decreases.
- They vibrate at a slower rate and move slowly.
- When the intermolecular attractive forces are strong enough to hold the particles in a fixed and orderly arrangement, liquid is changing into solid.
5. Sublimation
(a) Sublimation is a process in which a substance changes directly from solid to vapour phase without passing through the liquid state.
(b) Example:
 |
| Solid CO2 (Dry ice) |
| Iodine |
- Naphthalene
6. Deposition
(a) Deposition is a process in which a molecule changes directly from vapour to solid phase without passing through the liquid state.
(b) Example:
 |
| Snow |
7. Types of solid
(a) Crystalline solid

- Particles (atoms, ions or molecules) are arranged in a regular repeating geometric arrangement.
- Formed when a saturated liquid is cooled slowly.
- Has a sharp melting point.
- Example:
| Sugar |
 |
| Salt |
(b) Amorphous solid

- Particles have no well-defined order structure (has random structure).
- Formed when a saturated liquid is cooled rapidly.
- Has no definite melting point. Occurs over a range of temperature.
- Example:
| Glass |
| Synthetic solid |
 |
| Arrangement of atoms in crystalline and amorphous solid |
8. Types of crystalline solid
(a) Ionic solids
- Consist of ions (cation & anion) held together by ionic bonds.
- Example: Ionic salt (NaCl, KBr)

(b) Molecular covalent solids
- Composed of molecules held together by intermolecular forces (van der Waals and/or hydrogen bonds).
- Example:

Water (H2O)
 |
| Iodine (I2) |
 |
| Ammonia (NH3) |
(c) Giant covalent solids
- Large molecule.
- Composed of atoms linked together by covalent bonds.
- Example:
 |
| Diamond |
 |
| Graphite |
 |
| Quartz |
(d) Metallic solids
- Has a closely packed structure.
- Composed of atoms of the same metal linked together by metallic bond.
- Example: Metallic elements

1 comment:
As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. Today, Alfa Chemistry employs more than 200 full time staff, 1-ethyl-2,3-dimethylimidazolium nitrate
Post a Comment