The total pressure of a mixture of non-reacting gases is the sum of the partial pressures of all the gases present in the mixture at constant volume and temperature.
Ptotal = PA + PB + PC + ...
where Ptotal = total pressure of a mixture of non-reacting gases
PA, PB and PC = partial pressure of gases A, B and C in the mixture
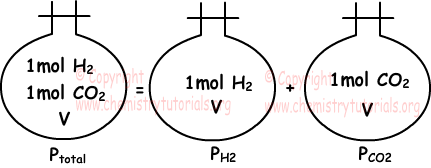
Partial pressure is the pressure that a gas would exert if it was present alone in the container.
From ideal gas equation,
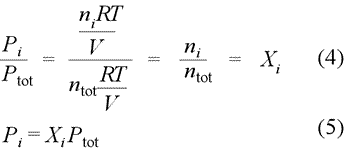
where Pi = partial pressure of gases
Xi = mole fraction of gases
Ptot = total pressure of a mixture of non-reacting gases
2. Collection of a gas by the displacement of water

When a gas is collected "over water", a mixture of the particular gas and water vapour is collected. By raising and lowering the collecting tube until the water level inside and outside the tube is the same, the pressure inside the collecting tube is exactly equal to the atmospheric pressure.
From Dalton's law of partial pressure,
No comments:
Post a Comment