If a system at equilibrium is subjected to a change of concentration, pressure or temperature, the system will shift its equilibrium position in such a way to reduce the effect of the disturbance.
2. Factors affecting the system at equilibrium
(a) Change in concentration of reacting species
Consider a reaction: 3 H2 (g) + N2 (g) ↔ 2 NH3 (g)
If H2 is added to a system at equilibrium, the equilibrium position will shift to right in order to consume the H2 added to the system. As a result, the concentration of H2 and N2 decreases, while the concentration of NH3 increases.
However, the value of equilibrium constant (K) remains constant (unchanged). The value of K calculated when the equilibrium is reestablished equals to the value of K at the initial equilibrium.

(b) Change in pressure (or volume)
- Signifcant only for system involving gaseous components.
- It can occur by changing the volume of the reaction vessel or adding inert gas (at constant pressure and at constant volume).

Consider a reaction: 3 H2 (g) + N2 (g) ↔ 2 NH3 (g)
(4 mol) (2 mol)
If the volume of the reaction vessel is decreased, it increases the pressure of the system. According to Le Chatelier's principle, the system will respond by shifting its equilibrium position to decrease the pressure of the system. The equilibrium position shifts to right, which has fewer number of moles of gaseous molecules. It is because fewer number of moles of gaseous molecules will exert lower pressure and thus the pressure of the system can be decreased.

Conversely, if the volume of the reaction vessel is increased, it decreases the pressure of the system. According to Le Chatelier's principle, the system will respond by shifting its equilibrium position to increase the pressure of the system. The equilibrium position shifts to left, which has more number of moles of gaseous molecules. It is because more number of moles of gaseous molecules will exert higher pressure and thus the pressure of the system can be increased.
(ii) Adding inert gas at constant volume
The total pressure of the system increases if an inert gas (such as Argon) is added. However, the partial pressure of each of the reacting gases remains constant (or unchanged), since number of mole (n) and volume (V) remain constant. The reaction quotient (Q) equals to K. Thus, the system is still at equilibrium and the equilibrium position remains unchanged.

(iii) Adding inert gas at constant pressure
The partial pressure of each of the reacting gases decreases when inert gas is added at constant pressure (since total number of moles of gases molecules increases). Consequently, the concentration or number of moles of gases per unit volume decreases.
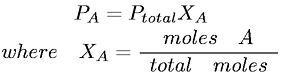
According to Le Chatelier's principle, the system will respond by shifting its equilibrium position to increase the number of moles of gases molecules (or concentration) of the system. Thus, the equilibrium position shifts to left, which has more number of moles of gaseous molecules.
(c) Change in temperature
Consider a reaction:
If the temperature of the system is increased, the system will respond by shifting its equilibrium position to decrease the temperature of the system according to Le Chatelier's principle. The equilibrium position shifts to left in order to consume (or absorb) the added heat. Thus, more N2 and H2 are produced, while NH3 is consumed. The value of K decreases.
For exothermic reaction, heat is considered as a product:
3 H2 (g) + N2 (g) ↔ 2 NH3 (g) + heat
Consider a reaction:
If the temperature of the system is increased, the system will respond by shifting its equilibrium position to decrease the temperature of the system according to Le Chatelier's principle. The equilibrium position shifts to right in order to consume (or absorb) the added heat. Thus, more CO is produced, while CO2 and C is consumed. The value of K increases.
For endothermic reaction, heat is considered as a reactant:
CO2 + C + heat ↔ 2 CO
Summary:
The value of K changes with the change in temperature.
(d) Catalyst
- Catalyst is a substance that speeds up a reaction by providing an alternative pathway which has lower activation energy.
- It increases the rate of forward and reverse reaction to the same extent (shorten the time to achieve equilibrium for a reaction).
- It has no effect on the equilibrium position and the value of equilibrium constant (K).

3. Industrial application on the chemical equilibrium
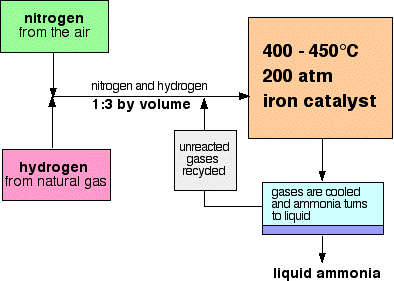
Haber process (synthesis of ammonia, NH3)
(a) Method to maximise the yield of ammonia:
- Add nitrogen (N2) and hydrogen (H2) to the system.
- Remove ammonia (NH3) from the system.
- Increase the pressure of the system (or decrease the volume of the system)
- Decrease the temperature of the system
(b) In reality, it is impractical and uneconomical if:
- the amount of reactant (N2 and H2) is too high.
- the pressure of the system is too high since it affects the cost and safetiness of the reaction.
- the temperature of the system is too low since it affects the rate of reaction.
(c) The optimum conditions for the reaction:
- Temperature: 450 - 500oC
- Pressure: 200 - 1000 atm
- Catalyst: Iron
4 comments:
Thank you sir.. it was useful :)
situs terpercaya agen sabung ayam
[img]https://i.imgur.com/03ce76Z.jpg[/img]
Bonus Next Deposit 5%
1. Promosi ini hanya berlaku untuk Member yang telah terdaftar di Museumbola
2. Minimal deposit untuk claim bonus 100.000
3. Maximal bonus yang diberikan 3.000.000
4. Bonus diberikan hanya 1 kali dalam sehari ( Wajib Claim )
5. Bonus berlaku untuk semua game yang tersedia di Museumbola
DEPOSIT BANK LOCAL :
BCA - MANDIRI - BNI - BRI - DANAMON - JENIUS BTPN DLL
DEPOSIT E-CASH :
OVO - DANA - LINKAJA - GOPAY - SAKUKU
DEPOSIT PULSA (DEPOSIT NON RATE/ALIAS TANPA POTONGAN) :
-> TELKOMSEL
-> XL / AXIS
Kontak dan Link
-> Link 1: https://bit.ly/2Wwc3s2
-> Link 2: https://bit.ly/2KWZEbt
-> Wa: +6283157394921
NB : HATI-HATI PENIPUAN MENGATAS NAMAKAN MUSEUMBOLA , SILAHKAN HUBUNGI KONTAK YANG TELAH KAMI INFO DAN VALID
TERIMA KASIH.
PROMO SPESIAL 100% SABUNG AYAM ONLINE | MENANG TARUHAN BERUNTUN -
Nikmati Promo Spesial Bonus 100% Khusus untuk Taruhan Sabung Ayam Onlie yang di siarkan secara Live (Langsung) dari Arena yang ada di Negara Filipina !
Pertandingan di liput secara live oleh kru proffesional dari Laga Tournament yang di adakan di negara tersebut ! Minimal Deposit hanya IDR 50.000,- Dan Untuk Taruhannya minimal IDR 20.000,- Saja
Dapat di tonton melalui Aplikasi Khusus yang dapat di download dan di Instal di Smartphone Android / iOS kesaangan anda !
Download Aplikasi Sabung Ayam Livenya sekarang juga ! Klik Di sini <<<===
Tersedia :
» Sabung Ayam S128
» Sabung Ayam SV388
Menerima Transakdi Deposit & Withdraw Menggunakan OVO | GOPAY | LINKAJA | DANA | PULSA dan SEMUA JENIS REKENING BANK DI INDONESIA.
Untuk Informasi selengkapnya, Hubungi Kontak Cs kami yang online 24 Jam dibawah ini :
» Nomor WhatsApp : +62812-2222-995
» ID Telegram : @bolavitacc
» ID Wechat : Bolavita
» ID Line : cs_bolavita
Post a Comment