German chemists have shown that it's possible to turn off aromaticity with a blast from a laser beam. Tuning the frequency of the laser excites electrons from the ground state to a non-aromatic state of alternating single and double bonds.
Inga Ulusoy and Mathias Nest at the Technical University Munich, performed the work as part of a larger project in Nest's lab. 'This is the first step in a broader project to control the reactivity of molecules in general,' Nest explains. 'The question we ask ourselves is, "Can we use a laser pulse to control the reactivity of a system?" and one of the easiest things for us to try [to control] is aromaticity, because we are theoreticians... so we decided to start with something small.'
'The idea is so simple that one can be angry that one didn't have it oneself,' says Jörn Manz, who works on laser control of reactions at the Free University of Berlin. He describes the work as a new twist in the field of laser control and 'a little ingenious'.
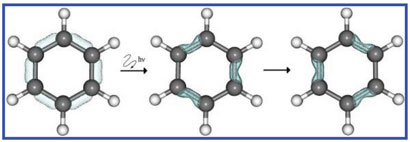 |
| Carefully tuned laser pulses can temporarily destroy benzene's aromaticity giving it a structure similar to that proposed by Kekulé |
As every student of chemistry learns, benzene is a highly stable compound with six equal bond lengths and delocalised pi electrons in rings above and below the plane of the molecule. However, there are several different definitions of aromaticity. Ulusoy and Nest decided to work with the definition of benzene as having six-fold electronic symmetry.
The pair identified non-aromatic states of benzene that do not satisfy this definition - one with different electron density on alternate atoms, and one with different electron density on alternate bonds. They then calculated the path and energy required to get to these states.
The non-aromatic states are actually a superposition of the ground state and the first or second singlet state, but a direct transition to either of the two excited states is forbidden by symmetry, so instead an alternative path had to be found via several other excited states. Ulusoy then put a trial laser pulse into her calculations and iteratively optimised the properties of the pulse to excite half of the ground state to one of the intermediate levels, and from the there to the final state.
One of these new states should look very familiar to anyone with a passing knowledge of chemistry. 'The state that we created corresponds to the cyclohexatriene molecule, where we have alternating bond orders of one and two,' says Manz. 'That was our practical definition of a non-aromatic state.' However, the molecule is not quite a true cyclohexatriene - the electron density moves so fast that the atoms can't keep up and the bond lengths are still all equal, much like Kekulé's resonance structures of benzene.
As a first step, Nest admits he is a long way off his final aim of firing laser pulses at compounds so that new reactions can be controlled and performed. However, Manz says he thinks this work should inspire further work in this area.
Source: Royal Society of Chemistry
No comments:
Post a Comment