Scientists in China have made a biofuel cell that harvests energy from soft drinks such as iced tea and juices.
Energy supply is a hot topic, explains Shaojun Dong, who made the cell with her team at the Chinese Academy of Sciences in Beijing. Biofuel cells (BFCs) convert chemical energy into electrical energy. They are cheap and active at room temperature and near-neutral pH, and show promise for use in green technology. Enzymatic BFCs that use an enzyme to convert sugar energy into electrical energy can be made into portable power sources and implantable medical devices as they generate more power than other BFC types.
BFCs need to be miniaturised and have access to an abundant fuel source to be able to power small electronic devices. Soft drinks are cheap and widely available, Dong explains. Just 1 mL of a drink could allow a fuel cell to provide electrical energy for over a month.
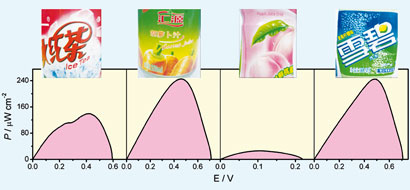
The group's enzymatic BFC contains a bioanode and biocathode made from carbon fibre microelectrodes, which were modified with single-walled carbon nanohorns - carbon nanotubes shaped like horns - to enhance electron transfer. The bioanode was further modified with the enzyme glucose dehydrogenase (GDH). With the help of an enzyme co-factor, which assists in enzymatic reactions, the GDH-modified bioanode oxidised glucose present in the soft drinks. The biocathode was modified with the enzyme bilirubin oxidase, and reduced oxygen in the air to water. The electric current created by these two reactions can be used to power devices.
Dong points out that other BFCs that harvest energy from fruit juice need purification steps in order to work. 'In our system, no complicated process is needed; supermarket soft drinks can be used directly,' she says.
Itamar Willner, an expert in bioelectronics at the Hebrew University of Jerusalem, Israel, says that using carbon nanohorns to immobilise GDH is an interesting idea. 'The high surface area of the bioanode nanocomposite leads to a relatively high power output,' he says. He adds that future devices will need some improvement, since the NAD+ co-factor will need to be covalently binded to the electrode material.
Dong concludes that the team is working to improve the bio-compatibility and long-term stability of implantable miniature BFCs for human beings and animals.
Source: Royal Society of Chemistry
1 comment:
In enzymology, a bilirubin oxidase (EC 1.3.3.5) is an enzyme that catalyzes the chemical reaction 2 bilirubin + O2↔ 2 biliverdin + 2 H2O. Thus, the two substrates of this enzyme are bilirubin and O2, whereas its two products are biliverdin and H2O. This enzyme belongs to the family of oxidoreductases, bilirubin oxidase
Post a Comment